
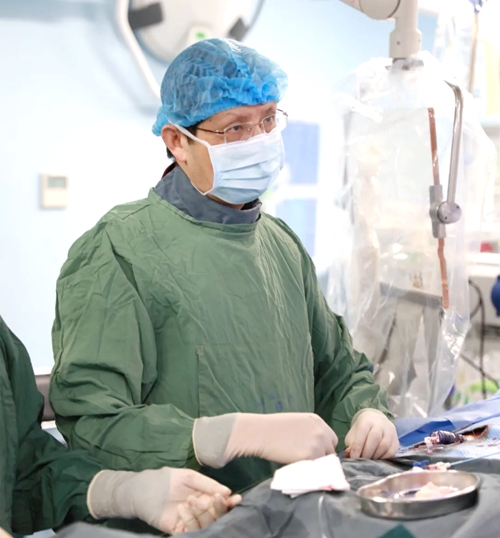
 PROF. WANG IN PROCEDURE
PROF. WANG IN PROCEDURE
SAHZU Hospital President and Heart Center Chair Prof. WANG Jian'an and his team completed first two TAVR cases in China using Edwards Sapien 3, the first kind of balloon-expandable transcatheter aortic valve system that has attracted much attention since its approval by the Chinese FDA. This long-awaited official clinical application indicates that interventional cardiology in China has moved to the next page of TAVR device development.
Edwards Sapien 3 is the first balloon-expandable transcatheter aortic valve system that enters China. It uses cobalt chromium alloy as its metal valve frame, exerting powerful force to the radial support that is more suitable for treating severely calcified heart valves. The artificial valve uses bovine pericardium tissue and its polyethylene terephthalate outer skirt can minimize paravalvular leak. This TAVR system has the biggest clinical evidence data with its over 25,000 enrolled patients worldwide. 455,00 patients have been implanted with the Sapien series of transcatheter aortic valve systems.
Author: | Reviewer: | Editor: | Source: | Date:2020-10-20 | Views:![]()
88 Jiefang Road,Shangcheng District Hangzhou,China, 310009
1511 Jianghong Road,Binjiang District Hangzhou,China, 310014
300 Yuanju Road, Shangcheng DIstrict, Hangzhou
456 Qidi Road, Xiaoshan District, Hangzhou, China
1 Xihu Avenue, Shangcheng District, Hangzhou
Zijingang Campus of Zhejiang University, 866 Yuhangtang Road, Xihu District, Hangzhou
Please call +86-571-8971 3988 (8am-5pm, Monday through Friday)
Make an appointment online

The Second Affiliated Hospital
Zhejiang University School of Medicine
88 Jiefang Road, Hangzhou, China
+86-571-8731 5108
iao_sahzu@zju.edu.cn
The Needs of Patients and Customers Come First.
The Second Affiliated Hospital Zhejiang University School of Medicine
All Rights Reserved.

