

Tumor-associated macrophage (TAM) reprogramming is a promising therapeutic approach for cancer immunotherapy; however, its efficacy remains modest due to the low bioactivity of the recombinant cytokines used for TAM reprogramming. mRNA therapeutics are capable of generating fully functional proteins for various therapeutic purposes but accused for its poor sustainability. Therefore, improving the sustainability of mRNA delivery is important for the development of effective mRNA-based therapeutics.
Recently, Chair of SAHZU Orthopedics Prof. YE Zhaoming collaborated with SAHZU Orthopedic Surgeon Prof. YU Xiaohua and Prof. CUI Wenguo, Ruijin Hospital of Shanghai Jiao Tong University School of Medicine, in pioneering the cytokine efficacy recovery system (CERS), inspired by kinetic energy recovery systems (KERS) in hybrid vehicles.
Their findings were published on Advanced Materials under the title of KERS-inspired Nanostructured Mineral Coatings Boost IFNγ mRNA Therapeutic Index for Antitumor Immunotherapy [1] with SHAO Zhenxuan, CHEN Liang, and ZHANG Zengjie as the co-first authors, and Prof. YE Zhaoming, Prof. YU Xiaohua and Prof. CUI Wenguo as the co-corresponding authors. (Click to access the article)
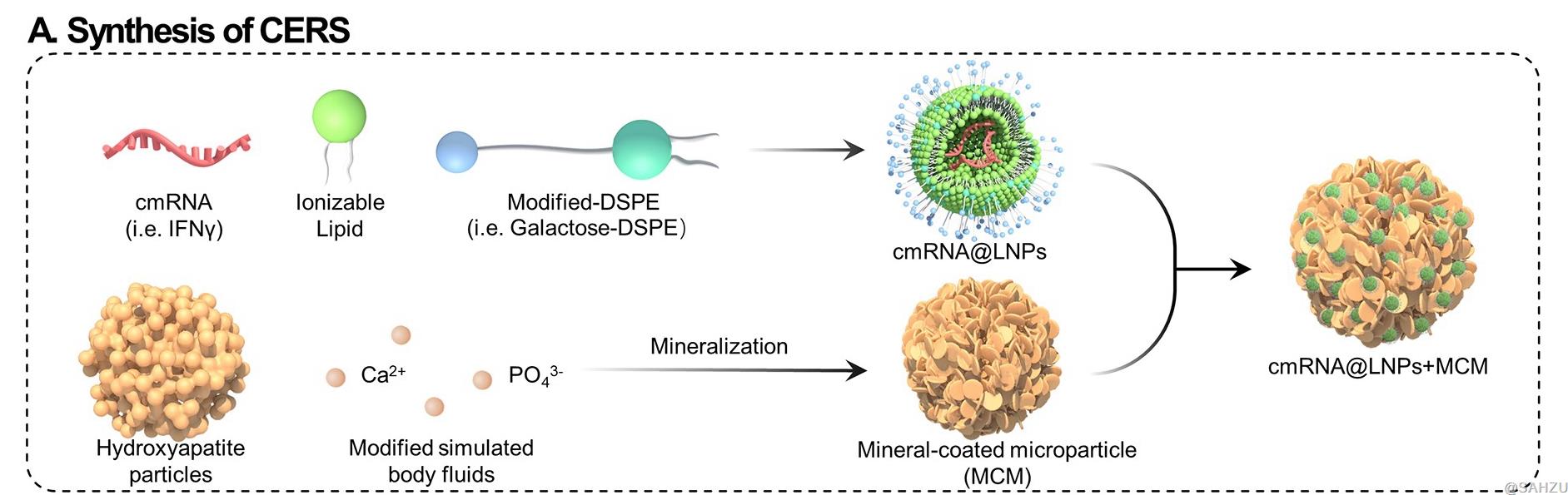
Figure A: Schematic illustration of CERS, which consists of surface-modified liposome nanoparticles (LNPs) loaded with mRNA and mineral-coated microparticle (MCM).
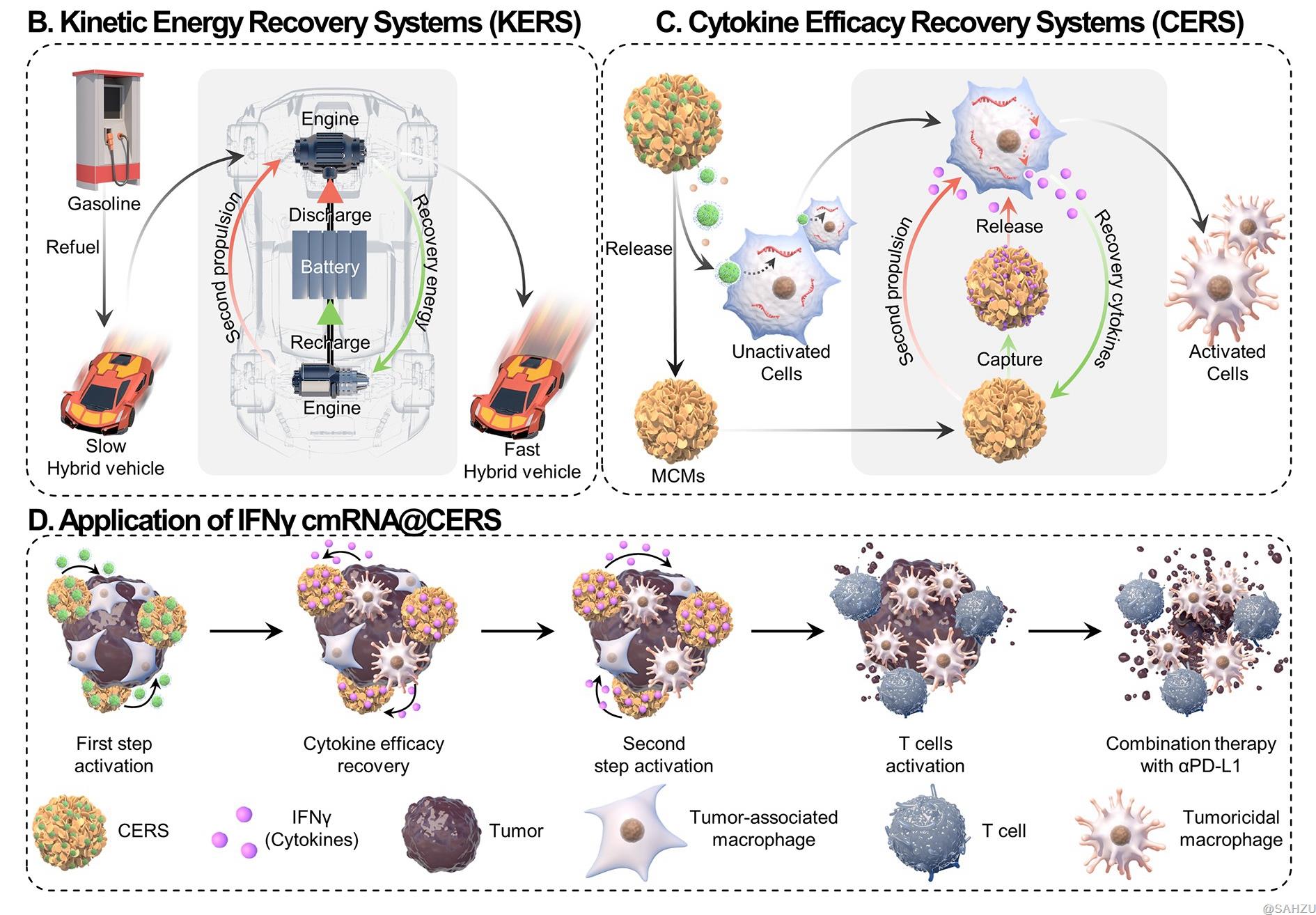
KERS is a power control system that improves fuel efficacy by storing energy during braking and subsequently releasing recycled kinetic energy for acceleration (Scheme B). Inspired by KERS, researchers designed CERS to substantially augment the therapeutic index of mRNA-based agents, not only by improving the mRNA expression, but also by recycling the expressed cytokines via an energy recovery system for hybrid cars.
To achieve this, mineral-coated microparticles (MCMs) are employed as mRNA delivery vehicles in CERS, as MCMs were previously reported to be able to both effectively improve transgene expression via secreted mineral ions [2] and capture expressed protein in situ via nanostructured mineral coating [3]. In response to the slightly acidic environment of the tumor, the MCMs then re-release the captured cytokines to extend the therapeutic window of the cytokines. Liposome nanoparticles (LNPs) of CERS are also modified to increase the mRNA delivery precision, as the surface-modified LNPs readily bind to target cells [4] (Scheme 1C).
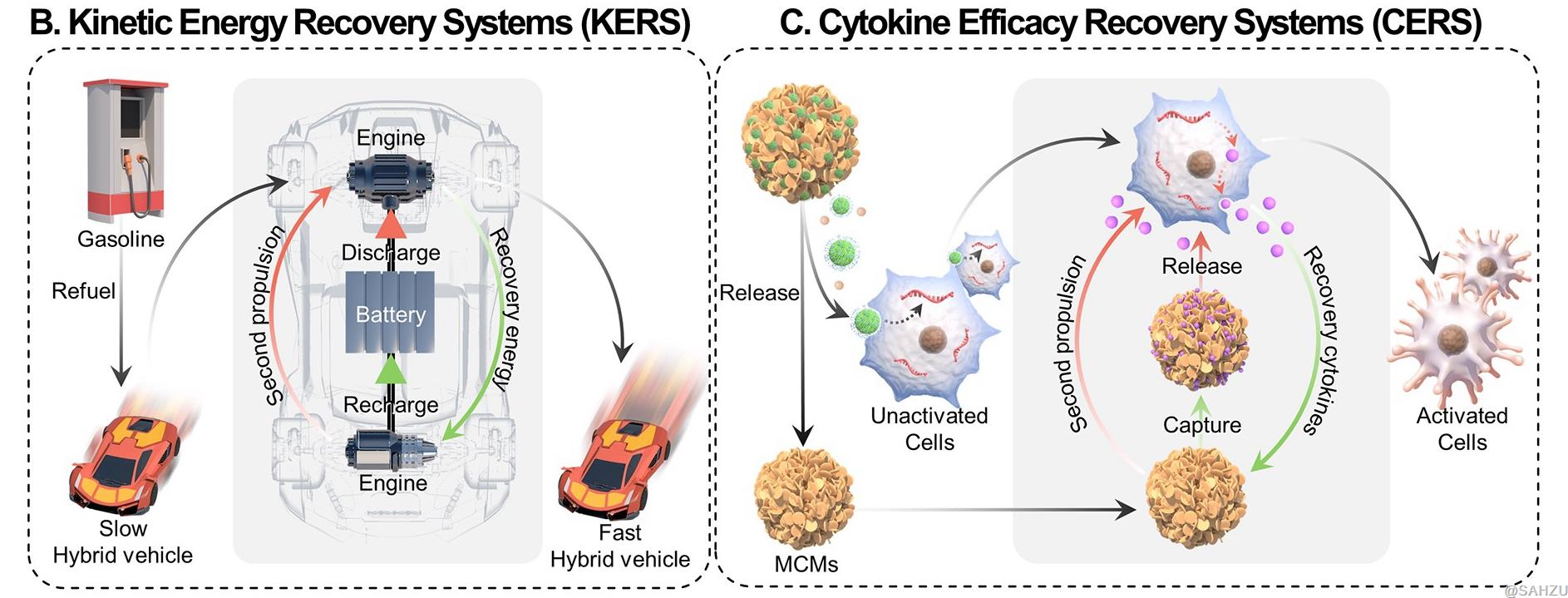
Figure B-C: (B) Schematic illustration of KERS, which is to make full use of kinetic energy by recovery. (C) Schematic illustration of CERS, mimicking the KERS for fully exploit cytokine efficacy: ① the mRNA enters effector cells through LNPs and translates into cytokines, and soluble mineral ions (such as Ca2+) from MCMs and surface modification of LNPs increase mRNA delivery efficiency; ② a part of cytokines stimulates target cells through receptors, and another part of unworked cytokines is captured by MCMs in CERS and stabilized against denaturation by the nanostructured mineral coatings; ③ cytokines are re-released and stimulate target cells as nanostructured mineral coatings gradually dissolves in the slightly acidic environment of the tumor.
Based on the above-mentioned efforts, the KERS-inspired CERS is hypothesized to drive more efficient mRNA-based production of therapeutic cytokines with the active capture and stabilization of labile cytokines, thereby greatly improving the efficacy and reducing the cost and side effects of cytokine therapy.
Using IFNγ as an example, it is demonstrated that CERS-mediated mRNA therapy induced a nearly 40% increase in translation efficiency and captured ~40% of labile IFNγ proteins, resulting in the doubling of IFNγ activity time. Compared with commercial rIFNγ, IFNγ expressed by mRNA@CERS exhibited ~42-fold higher biological activity.
When used for immunotherapy for osteosarcoma, CERS-mediated IFNγ mRNA delivery effectively reprogramed tumor-associated macrophages (TAMs) into tumoricidal macrophages and substantially inhibited tumor growth by inducing antitumor immunity. Interestingly, extremely malignant lung metastases even magically disappeared after receiving CERS-mediated IFNγ mRNA delivery combined with programmed cell death ligand-1 (PD-L1) antibody treatment (Scheme 1D).
Overall, inspired by KERS concept of hybrid electrical vehicles, CERS-mediated mRNA therapy via recovering cytokine efficacy provides a novel approach for efficient and sustained cytokines biotherapy, suitable for a wide range of medical applications.
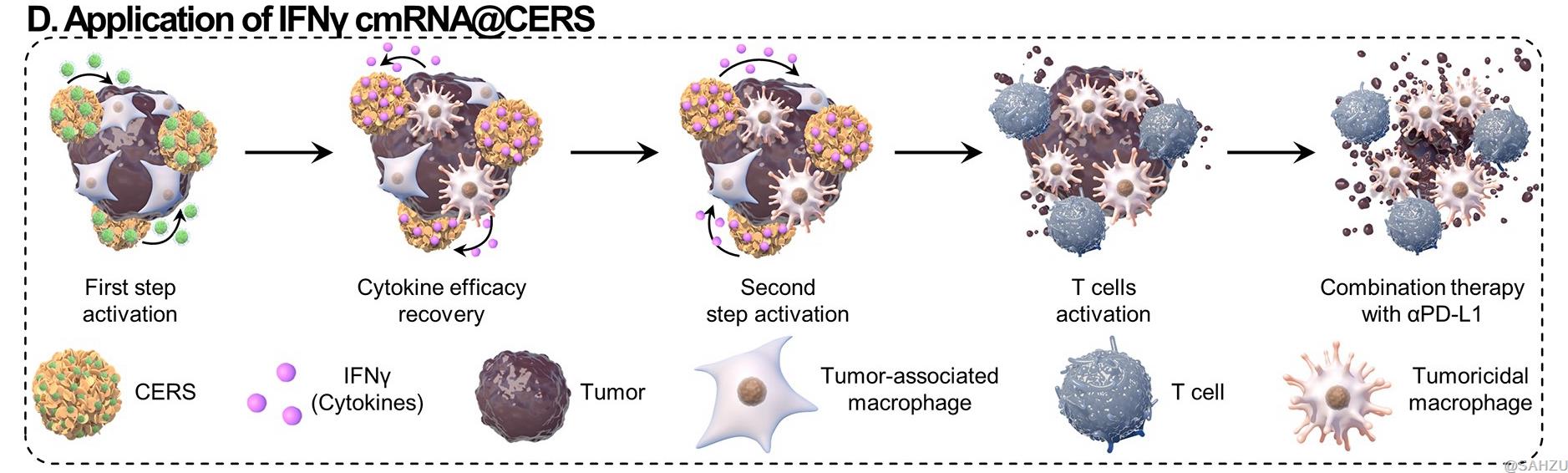
Figure D: CERS-mediated IFNγ chemically modified RNA (cmRNA) delivery expresses with high bioactivity and long duration, which effectively activates effector cells, thereby inhibiting tumor cell proliferation and activating immune responses.
References:
[1] Z. Shao, L. Chen, Z. Zhang, Y. Wu, H. Mou, X. Jin, W. Teng, F. Wang, Y. Yang, H. Zhou, Y. Xue, Y. Eloy, M. Yao, S. Zhao, W. Cui, X. Yu, Z. Ye, Adv Mater. 2023, e2304296.
[2] G. Fontana, H. L. Martin, J. S. Lee, K. Schill, P. Hematti, W. L. Murphy, Mol Ther Nucleic Acids. 2019, 18, 455.
[3] a)X. Zhou, J. Chen, H. Sun, F. Wang, Y. Wang, Z. Zhang, W. Teng, Y. Ye, D. Huang, W. Zhang, X. Mo, A. Liu, P. Lin, Y. Wu, H. Tao, X. Yu, Z. Ye, J Nanobiotechnology. 2021, 19, 420; b)X. Yu, A. H. Biedrzycki, A. S. Khalil, D. Hess, J. M. Umhoefer, M. D. Markel, W. L. Murphy, Adv Mater. 2017, 29, e1701255.
[4] Y. Tang, Z. Tang, P. Li, K. Tang, Z. Ma, Y. Wang, X. Wang, C. Li, ACS Nano. 2021, 15, 18100.
Author: SHAO ZHENXUAN | Reviewer: LI JING | Editor: LI JING | Source: SAHZU ORTHOPEDICS | Date:2023-08-29 | Views:![]()
![]()
![]()
![]()