

Deep tissue infection is a common clinical issue and therapeutic difficulty caused by the disruption of the host antibacterial immune function, resulting in treatment failure and infection relapse.
In such case, therapeutic options are often limited and heavily rely on aggressive antibiotic chemotherapy, which may further causes substantial harm to host immune function and often yields unsatisfactory therapeutic outcomes. In view of this, the pursuit of novel approaches to combat infections holds paramount importance.
Host immune defense mechanisms can be roughly divided into the innate and adaptive arms, and an effective immune response requires an orchestrated cooperation of both arms. Upon infection, innate immunity is immediately activated to eliminate invasive pathogens. However, invasive pathogens, such as Staphylococcus aureus, have evolved sophisticated mechanisms to evade antibacterial strategies. They are able to hide inside the host cells and can manipulate their biology even after appropriate treatment, resulting in a locoregional immunosuppressive state that leads to an inadequate response to conventional anti-infective therapies.
Recently, Chair of SAHZU Orthopedics Prof. YE Zhaoming and his team presented an innovative strategy to harness host immune responses for the treatment of deep tissue infections. Their research article titled An on-demand collaborative innate-adaptive immune response to infection treatment was published on Advanced Materials on July 31th .(Click to access the article)
CHEN Liang, SHAO Zhenxuan and ZHANG Zengjie are the co-first authors of this article. Prof. YE Zhaoming and Prof. XU Jianbing are the co-corresponding authors of this article.

Figure 1. Schematic illustration of the reversal of immunosuppressive infectious microenvironment by nanoparticle-armed infection-associated macrophages (IAMs), achieving infection treatment and preventing relapse via autogenous immunity remodeling.
Macrophages, as essential components of the innate immune system, are monocyte-derived cells that form a bridge with the adaptive immune arm and regulate host immune homeostasis. They recognize and perform sequential engulfment of bacteria, and process antigen presentation to the adaptive immune cells after intracellular destruction of pathogens in the presence of the reactive oxygen species (ROS).
The research team found that inadequate ROS levels in infected macrophages led to intracellular survival of invading pathogens, skewing the macrophage response from the pro-inflammatory M1 phenotype towards an anti-inflammatory M2 phenotype.
Resembling those of tumor-associated macrophages (TAMs) that play pivotal roles in the formation of the complex tumor immunosuppressive microenvironment, the compromised infected macrophages would cause a similar critical failure in priming of adaptive immunity, subsequently leading to immune exhaustion and infection development.
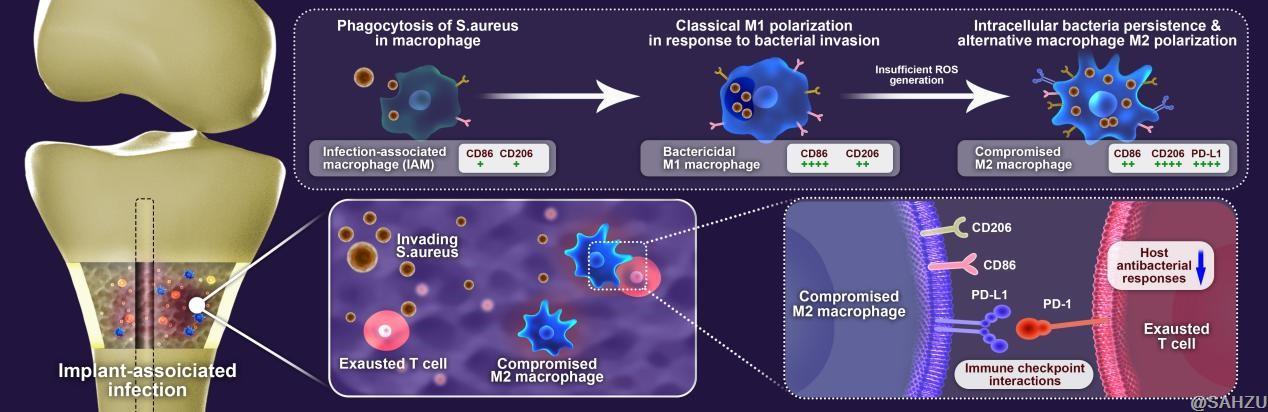
Figure 2. Diagram of compromised macrophages with intracellular bacteria and their actions to adaptive T cells.
Based on the intrinsic antibacterial process of macrophages, the researchers aimed to utilize the metabolic alterations occurring in macrophages infiltrating infection sites and described an artificially inducible collaborative innate-adaptive immune response for immune recovery and precise infection treatment. Thus, they constructed a regulatory system (MφL@BSA@Man-ICG NPs) for on-demand collaborative innate-adaptive immune responses for infection treatment.
Specifically, a pH-responsive hybrid membrane shell (MφL) by integrating the murine macrophage line J774.1A membranes and pH-responsive vesicles, which were then encapsulated with the functional core BSA@Man-ICG to obtain biomimetic nanoclusters. MφL was selected as the carrier because of its superior bacteria-targeting ability, conferred by the macrophage membrane, whereas its pH-responsive release nature realized spatiotemporal cargo unloading at the infection site.
Indocyanine green (ICG), an FDA-approved diagnostic reagent, was selected as an ROS generator, which was mannose-functionalized for targeted delivery to macrophages (denoted as Man-ICG) and self-assembled into bovine serum albumin (BSA) nanoparticles based on noncovalent interactions. The above characteristics of these materials serve as the basis for this cell-engineering antibacterial strategy, enabling infection-associated macrophages to be reprogrammed as required.
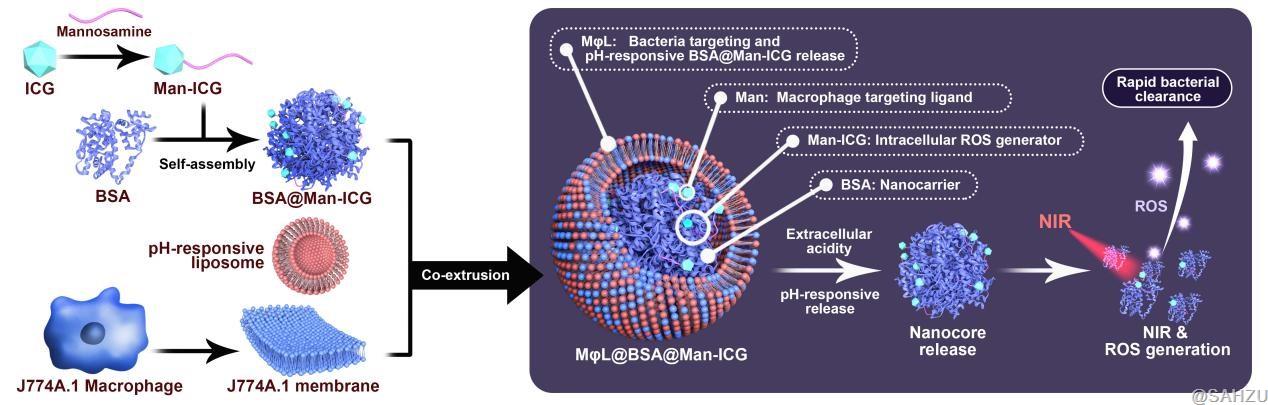
Figure 3. Schematics of MφL@BSA@Man-ICG construction and its potential mechanism for antibacterial effect.
An instant ROS burst within nanoparticle-armed macrophages induced via in-situ photodynamic therapy eliminated residual pathogens. Additionally, ROS can activate the NF-κB signaling axis, which promotes macrophage M1 repolarization and initiates macrophage-central antibacterial immune responses. Moreover, they observed enhanced antigen presentation and T-cell priming by in-situ armed macrophages, which resulted in highly activated production of inflammatory factors to reinforce a robust collaborative innate-adaptive antibacterial immunity.
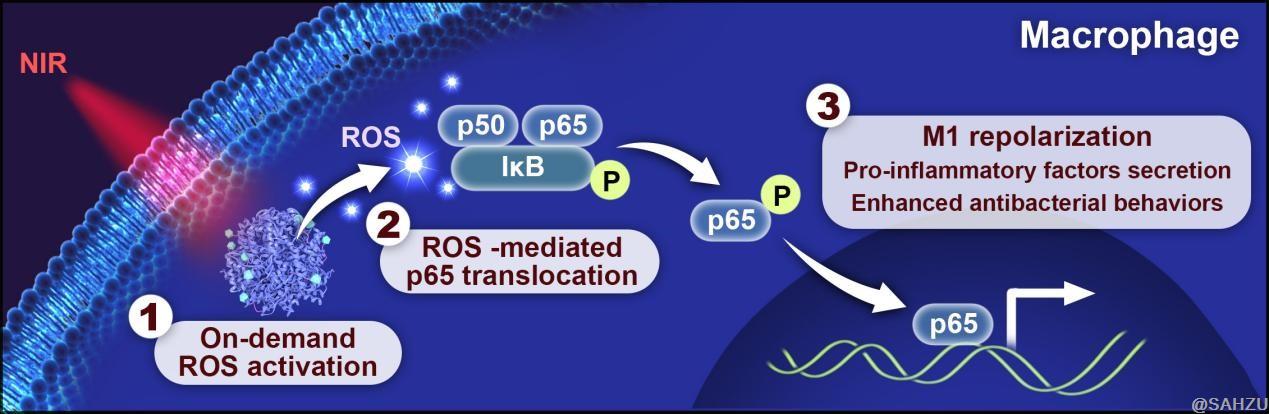
Figure.4 Schematic diagram of armed-macrophage M1 repolarization and their actions in bacterial clearance.
In conclusion, this study demonstrated the crucial role of IAMs in host defense against bacterial infections. IAM-directed host innate adaptive immune responses, based on ROS generation by intracellular BSA@Man-ICG, achieved S. aureus clearance and prevented bacterial relapse. Nanoparticle-armed macrophages, reminiscent of CAR-M cells, represent a simple cell-engineering strategy. It solves the ROS regulation problem that leads to intracellular bacterial survival and cellular compromise and consolidates the vital role of infectious immune microenvironment remodeling in bacterial infection treatment.
Prof. YE pointed out the significance of this finding, "Our work provides a scientific basis for a novel antibacterial strategy based on consolidation of host intrinsic immunity without the use of gene editing and antibiotic administration, which has strong potential in clinical applications."
Author: CHEN LIANG | Reviewer: LI JING | Editor: LI JING | Source: SAHZU ORTHOEPDICS | Date:2023-08-29 | Views:![]()
![]()
![]()
![]()